Ialuril Prefill 50 ml pre-filled syringes
Ialuril Prefill Fertspr 50 ml
-
255.73 USD
You save 0 / 0%
Buy 2 and save -10.24 USD / -2%

- Availability: In stock
- Distributor: INS. BIOCHIMIQUE SA
- Brand: Ialuril
- Product Code: 7746702
- EAN 8033638952627
Ingredients:
Description
Medical device
composition
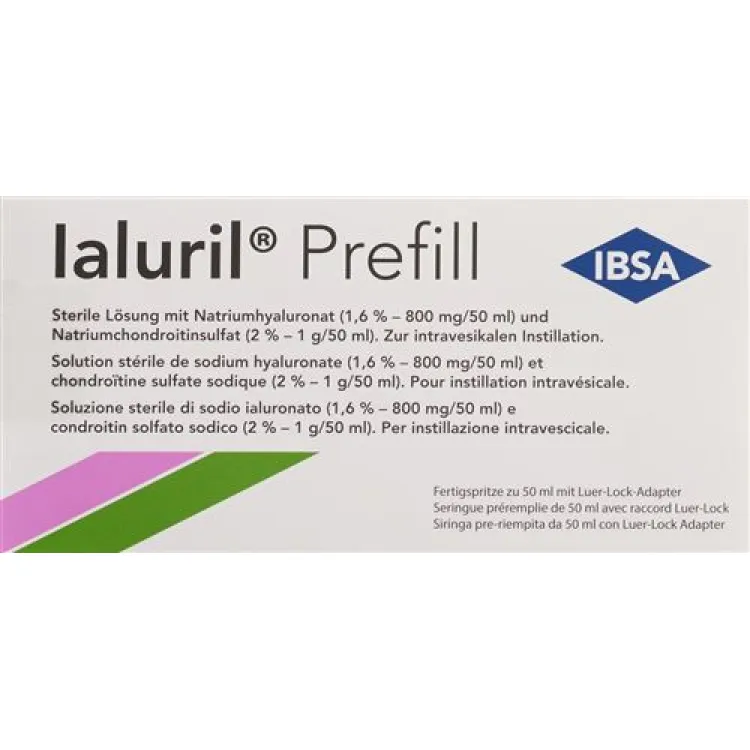
Each 50 ml prefilled syringe of Ialuril Prefill contains: water, calcium chloride, hyaluronic acid sodium salt, sodium chondroitin sulfate.
Galenic form and amount of active ingredient per unit
Prefilled 50 ml syringe with Luer lock adapter and Ialu adapter.Sterile solution containing sodium hyaluronate (1.6% - 800 mg/50 ml), sodium chondroitin sulfate (2% - 1 g/50 ml) and calcium chloride (0.87% - 440 mg/50 ml).
Indications/applications
Ialuril Prefill is indicated for the restoration of the glycosaminoglycan layers (GAG) of the vesical urothelium, in cases where their loss can cause frequent and recurring complaints (such as cystitis of various etiologies).Ialuril Prefill is also indicated in those cases in which the loss of the glycosaminoglycan layers (GAG) is associated with forms of chronic inflammation in which the composition and integrity of these layers appear to be variably damaged.
Dosage/Application
For intravesical instillation.Each prefilled syringe is intended for one patient only.
Frequency of application
It is recommended to instill the contents of 1 prefilled syringe according to the following scheme:
- 1 instillation per week during the first month;1 instillation every two weeks during the second month.In the following months, 1 instillation per month is recommended until symptoms are stable, or as directed by a doctor.
Ialuril Prefill can be administered via a catheter or the Ialuadapter.The choice of administration method is based on medical opinion for each patient.
Application of Ialuril Prefill via a catheter
- After the patient has spontaneously urinated, the urinary bladder must be freed of all urine residue using a specially designed sterile catheter via the external urethral opening (meatus urethrae externus).Place the piston rod provided on the prefilled syringe and screw it tight.Attach the Luer lock adapter to the top of the prefilled syringe and connect it to the sterile catheter positioned in the urinary bladder.Slowly instill the entire contents of the disposable syringe into the urinary bladder via the catheter.Once the product has been instilled into the bladder, carefully remove the catheter with the syringe and discard.Leave Ialuril Prefill in the bladder for as long as possible (recommended minimum residence time 30 minutes).
Application of Ialuril Prefill via the Ialuadapter
- Before starting treatment, the patient is asked to urinate and ensure that the bladder is completely empty before instillation.Place the piston rod provided on the prefilled syringe and screw it tight.Mount the Ialuadapter onto the upper end of the prefilled syringe with a half-turn movement to ensure a stable hold.Slowly instill the entire contents of the disposable syringe into the bladder using the Ialu adapter.As soon as the product has been instilled into the bladder, carefully remove the Ialuadapter with the syringe and discard it.Leave Ialuril Prefill in the bladder for as long as possible (recommended minimum residence time 30 minutes).
Contraindications
To date, there are no known contraindications that can be attributed to the use of Ialuril Prefill.Ialuril Prefill must not be used if you are known to be hypersensitive to any of its components.
Warnings and PrecautionsThe administration of Ialuril Prefill via the catheter or the Ialuadapter must only be carried out by qualified personnel.
This procedure must be carried out under sterile conditions and with sensitivity, as the patient, for example if interstitial cystitis is present
- is particularly exposed to the development of bacterial cystitis, which worsens the symptoms of the present disease,complains of lower abdominal pain,deliberately urinated less often in order not to worsen the pain in the lower abdomen caused by urination (hypertonia of the muscles induced by the pain).
Wash hands with great care, if possible with an antibacterial cleanser, and then put on sterile gloves before proceeding with the preparation and administration of Ialuril Prefill.Carefully follow the instructions in the manual for performing an intravesical instillation.
Interactions
There are no known interactions of Ialuril Prefill with other drugs commonly used by patients with cystitis of various etiologies.
unwanted effects
There are no known side effects resulting from the use of Ialuril Prefill.Occasionally, patients may experience local reactions (irritation, burning) due to the instillation procedure, but these are not attributable to the Ialuril Prefill medical device.If any undesirable effects occur, treatment should be discontinued.
Properties/Effects
The urothelium is lined with a layer of polyanionic molecules consisting predominantly of glycosaminoglycans (GAG), a class of amino sugars that form an impenetrable and neutralizing protective barrier against the toxic and irritating substances present in urine (such as bacteria, microcrystals, proteins, ionic and non-ionic residues, etc.) and their systemic reabsorption.Of the GAGs that form this protective barrier, chondroitin sulfate and hyaluronic acid play a central role in the function of the barrier itself.Qualitative and quantitative fluctuations of these two GAGs at different levels inactivate the barrier effect and thus cause a series of conditions that can favor the occurrence of cystitis of different nature (e.g. interstitial cystitis, recurrent cystitis caused by infections, cystitis caused by substances used in cancer treatment). , radiation-induced cystitis, traumatic cystitis).Ialuril Prefill, a balanced combination of sodium hyaluronate, chondroitin sulfate and calcium chloride, can be functionally integrated into the barrier thanks to the effect of calcium chloride and thus restore its protective function.
Other information
Ialuril Prefill is steam sterilized.Ialuril Prefill is latex-free.Ialuril Prefill may only be used until the expiry date stated on the pack.Do not use the Luer Lock adapter if the package is opened or damaged.Do not use the Ialuadapter if the package is opened or damaged.Ialuril Prefill must not be used if the package appears to be opened or damaged.Ialuril Prefill must not be used if contamination or streaks are evident in the solution.Do not re-sterilize.Unused solution residues may no longer be used.Do not reuse to avoid any risk of contamination.Once opened, Ialuril Prefill must be used immediately and discarded after use.Store between 0-25°C and away from heat sources.Keep out of reach of CHILDREN.May only be given against a doctor?s prescription.
Sales company
IBSA Institut Biochimique SA, CH-6903 Lugano.
manufacturerLuer lock adapter
Primed Halberstadt Medizintechnik GmbH, Strasse des 20. Juli 1, D-38820 Halberstadt.
Ialu adapter
Dispomedicor Zrt, Füredi ùt 98, H-4032 Debrecen.
Ialuril Prefill prefilled syringe
IBSA Farmaceutici Italia Srl, Via Martiri di Cefalonia 2, I-26900 Lodi (LO).